Wellaholic Research: Tooth Whitening: What We Now Know
TL:DR Summary
- Abstract: The article summarizes current research on tooth whitening and its safety and efficacy.
- Background: Tooth whitening is a popular dental procedure that uses hydrogen peroxide to bleach stains on the teeth.
- Methods: The article reviews current reports in the literature on tooth whitening methods, including in vitro and clinical studies.
- Whitening Chemistry: The article explains how hydrogen peroxide reacts with organic and metal compounds that cause tooth stains and lightens their color.
- What’s new in bleaching research?: The article discusses new research on optimizing whitening procedures, reducing tooth sensitivity, increasing whitening persistence, and assessing risks such as tooth surface damage, demineralization, restoration degradation, and color change.
- Summary: The article concludes that tooth whitening is safe and effective when manufacturer’s protocol is followed, but there are risks that should be considered and monitored by the profession and users.
Abstract
Current research about tooth whitening shows that it is safe and effective when manufacturer’s protocol is followed, yet there are risks of which the profession and users should be aware. This update provides a summary of current research and assessment of the safety and efficacy of tooth whitening regimens.
Background
The Popularity of Tooth Whitening
Tooth whitening has become one of the most popular cosmetic dental procedures. The public has increasingly demanded whiter, more perfect smiles, and as a result, many choices for tooth whitening have become available.
Types of Tooth Whitening Products
These options include home-based products like toothpaste, gels, and films, as well as in-office systems where highly concentrated bleaching agents are applied under professional supervision.
New Research and Risks
While tooth whitening can be effective, there are also risks associated with the procedure. The profession and public have been aware of certain risks such as increased tooth sensitivity and gingival irritation. However, new research has shown that tooth whitening can also lead to tooth surface roughening and softening, increased potential for demineralization, degradation of dental restorations, and unacceptable color change of dental restorations. This new research has prompted efforts to optimize whitening procedures to reduce tooth sensitivity and increase the persistence of the whitening effect.
Methods
Current reports in the literature are reviewed that are related to the use of peroxide based whitening methods. These reports include in vitro studies for method optimization and mechanism as well as clinical studies on effects of various whitening regimens.

Whitening Chemistry
Tooth whitening is any process that lightens the color of a tooth. Whitening may be accomplished by physical removal of the stain or a chemical reaction to lighten the tooth color. Bleaching is defined here as the chemical degradation of the chromogens. The active ingredient in most whitening products is hydrogen peroxide (H2O2) which is delivered as hydrogen peroxide or carbamide peroxide. Carbamide peroxide is a stable complex that breaks down in contact with water to release hydrogen peroxide. Because carbamide peroxide releases hydrogen peroxide the chemistry of most tooth whitening is that of hydrogen peroxide.
Tooth stains consist of compounds that have color or darker shades called chromogens that are accumulated in the tooth (intrinsic) or on the tooth (extrinsic). Chromogens fall into two categories: large organic compounds that have conjugated double bonds in their chemical structure as shown in Fig 2a; and metal containing compounds. Bleaching of the organic compounds by hydrogen peroxide involves reacting with the double bonds to oxidize the double bond as shown in Fig 2b. This causes the chromogen to become a lighter colored compound. Bleaching of the metallic compounds is much more difficult; better esthetic options may be veneers, bonding, or crowns. There are some professional products that contain sodium hypochlorite (NaOCl) which reacts with the double bonds of the chromogen in much the same way as peroxide as shown in Fig 2c.

Different product types are marketed to address the particular stain to be removed. The broad categories include: cleansers such as smokers’ toothpastes which contain larger amounts of detergents and abrasives to aid in the removal of chromogens from the tooth surfaces; bleaches to react with the chromogens to lighten color; and products that have both increased cleansing and bleaching. More severe or complex stains are best lightened professionally, while over-the-counter products can be used for milder cases. The treatment time required for reaching the tooth whitening end point is dependent on the time of exposure and the concentration of bleaching compound. This end point is dependent on the type of whitening employed, usually 1 to 2 shades for cleansers and over-the-counter (OTC) gels, and more for professionally applied products.
What’s new in bleaching research?
New studies have shown that aggressive tooth bleaching can cause increased tooth sensitivity, changes in tooth microstructure, and restoration changes. Aggressive bleaching can chemically react with composite restorations, glass ionomer cements, sealants, and ceramic crowns, thus reducing their stability.
- In one in vitro study seven tooth colored restoration substrates including a nanohybrid composite, a microhybrid composite, a flowable composite, and a packable composite resin, along with a compomer, a glass ionomer cement and a sintered ceramic used for CAD\/CAM restorations were exposed to 40 % hydrogen peroxide gel following manufacturer’s instructions at either 25 °C or 37 ° C and placed in artificial saliva between treatments.5 All of the materials were found to have surface softening with greater softening observed at 37 °C except for the sintered ceramic. No substance loss was observed for any material. However, the conditions of the experiments did not include subsequent abrasion (toothbrushing with dentifrice) as would occur in vivo and thus loss of restorative material from the softened surfaces is possible.
- Another in vitro study looked at the effect of home bleaching on color change and translucency of resin composites. Five commercially available composites were treated for 14 days with either 10 % carbamide gel or 10 % hydrogen peroxide gel following manufacturer’s instructions. There was significant (and unacceptable) color change in all of the composites with either of the home-based treatments. They found no significant difference between the carbamide peroxide and hydrogen peroxide treatments in the color changes. Interestingly, there was no change in the resin composite translucency indicating that the color change was restricted to the surface of the resin composites.6
- A randomized clinical trial showed that in-office bleaching of restored teeth using a 35 % hydrogen peroxide product caused tooth sensitivity in all cases. There was significantly greater intensity of tooth sensitivity pain for teeth that had restorations than for sound teeth. It was concluded that in-office bleaching with 35 % hydrogen peroxide was effective for patients with restored teeth, however that a higher degree of pain was found for these patients, especially associated with the upper lateral incisors.7
The risk of tooth structure damage has received attention by researchers who have evaluated the effects of aggressive tooth bleaching treatments on tooth structure and susceptibility to demineralization. Some of these studies have found that aggressive whitening treatments can change the surface integrity, microstructure of enamel crystals, and susceptibility to demineralization.
- In an in vitro study performed in our lab we tested five different bleaching products for erosion of human enamel. The five products included strips at 6.5 % hydrogen peroxide, and gels at 10 % and 22 % carbamide peroxide, 35 % hydrogen peroxide, and a sodium hypochlorite containing gel system. We followed the manufacturer’s instructions and measured the loss of enamel surface by polarized light microscopy. We found that although some surfaces were softened, no enamel erosion was found.8
- A new in vitro study conducted in Japan evaluated the susceptibility of a tooth to subsequent demineralization after a home based 10 % carbamide peroxide bleaching regimen compared to a more aggressive office-based 35 % hydrogen peroxide photoactivated regimen. Extracted teeth of color Vita shade A3 were bleached following manufacturer’s instructions until the shade was lightened to Vita shade A2 for all teeth. The teeth were then demineralized for two weeks and evaluated for mineral density loss. This study found that the surface integrity of the home-based treated teeth was altered such that significantly greater demineralization occurred in the home-based treated teeth than in the office-based treated teeth. The authors postulate that the peroxide in the office based gel penetrates deeper into the enamel before it was activated by photoirradiation.9
- The effect of cold-light activated bleaching treatment on enamel surfaces in vitro was studied because there is concern that either heat (infrared) or ultraviolet light exposure of teeth and gingiva during office bleaching could cause pulp and/or gingival tissue damage.10 In this study the crystal and surface microstructure of dental enamel was evaluated after 35 % hydrogen peroxide bleaching with cold-light irradiation (group LP); 35 % hydrogen peroxide bleaching without cold-light irradiation (group P); a control group treated with what is identified as a silica dioxide catalyzing agent as a peroxide-free bleaching agent (control group NP); and a control group treated with the cold-light irradiation alone (control group L). The authors report that no color change or enamel crystal size change was observed in the two control groups, indicating that the cold-light treatment alone or the silica dioxide agent had no effect on the tooth structure. However, the groups that had 35 % hydrogen peroxide (with or without cold-light) did result in significant color change as well as significant decrease in crystal size and crystallinity. There was no significant difference in the degree of color change between the cold-light activated hydrogen peroxide and the hydrogen peroxide only groups. Additionally, surface roughness was observed to increase in all groups except the cold-light only control group. The authors conclude that the 35% hydrogen peroxide solution had a major demineralization effect on enamel surface and the cold-light had no significant increased or decreased effect on the demineralization or color change. The mechanism suggested for the surface demineralization is that during the bleaching procedure the pH of the whitening agent becomes more acidic and the hydrogen ions attack the enamel crystals, freeing calcium and phosphate ions from the enamel surface. Several important deductions can be made from this study: 1. Aggressive bleaching can lead to surface demineralization and reduction of enamel crystallinity; 2. Cold-light activation seems to have little effect on color change or tooth structure; and 3. Hydrogen peroxide reduces the pH on the tooth surface during treatment, leading to surface demineralization.
One of the common questions posited by patients is “How long will the treatment last?” It is difficult to predict the persistence of bleaching regimens because the patient may routinely expose their dentition to food or beverages which are known to stain teeth and would experience re-staining within a month. However, if the teeth are not exposed to chromogens such as coffee, red wine, cigarette smoke, then it would be reasonable to assume that whitened teeth could persist or up to a year. There are several in vitro and clinical studies on this topic.
- An in vitro study that looked at the color change of enamel, dentine, and combined enamel and dentin of bleached tooth samples found that the color was not stable over time with regard to lightness. However, yellowness did not return to baseline within 1 year.11
- Another in vitro study designed to evaluate the color stability of bleaching after light activation with either a halogen unit, laser, LED unit; or bleaching without light activation found that all the tested methods achieved good aesthetic results even 3 months after the end of the bleaching. Additionally, the authors found that light activation of the bleaching agent was not beneficial compared to bleaching alone and light activation made no difference in the color stability up to 3 months after bleaching.12
- In a recent clinical trial it was found that by following a regimen consisting of in-office bleaching for two sessions with a 1-week interval, followed by home bleaching once a month for 3 months gave more persistence in color change over a 6-month period than in-office bleaching alone.13 This later study shows that this regimen for whitening maintenance can extend the effectiveness of the whitening treatment.
Summary
When used following manufacturer’s instructions, hydrogen peroxide and carbamide peroxide based tooth whitening is safe and effective. However as with all dental therapies, there are risks, and practices should be tailored to the needs of each individual patient, based upon type and extent of staining, dietary habits, previous restorations and other intraoral conditions. Patients should be informed of the risks associated with tooth whitening and, if using agents a home, instructed for identification of adverse occurrences in order to seek professional help if needed. Supervision of the tooth whitening strategy by an oral healthcare professional will reduce the potential risks and optimize benefits of tooth bleaching.16
Source
- National Library of Medicine | J Evid Based Dent Pract. Author manuscript; available in PMC 2015 Jun 1.
- Tooth Whitening: What We Now Know
- Cristian Bersezio 1, Javier Martín 1, Pablo Angel 1, Jessica Bottner 1, Isidora Godoy 1, Francisca Avalos 1, Eduardo Fernández 2 3
GET IN TOUCH
Book Now Pay Later

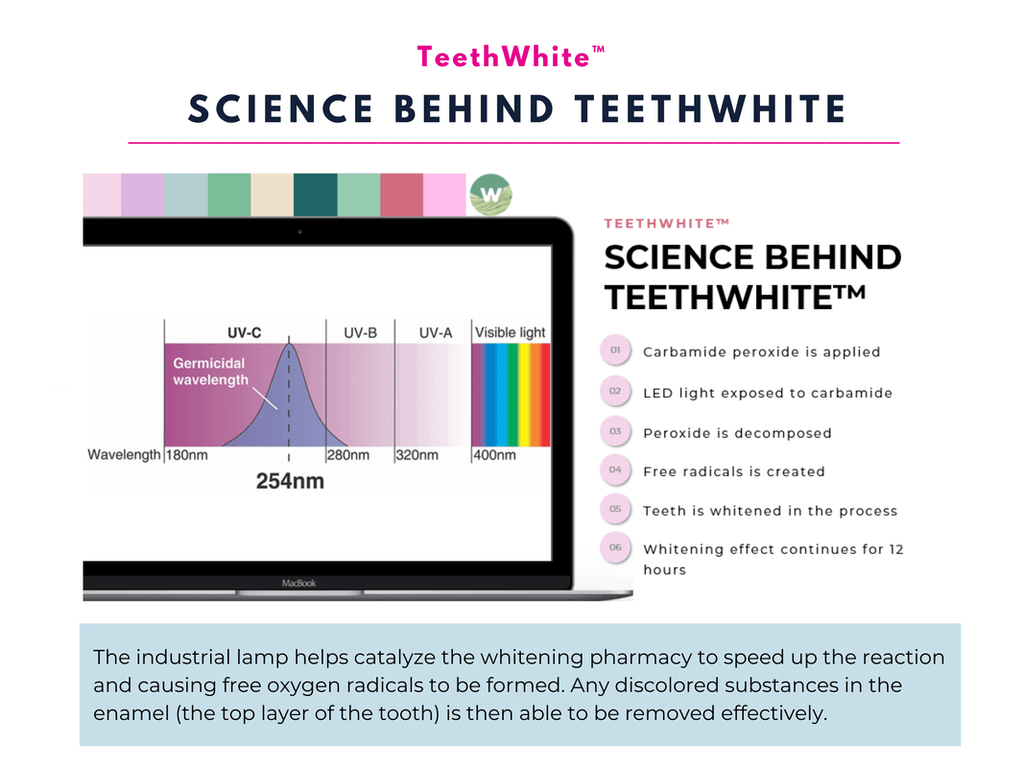
TeethWhite™ Teeth Whitening – Get Up to 12 Shades Whiter
- ⭐ Immediate Results. TeethWhite™ brightens your smile up to 12 shades in a single session, offering an instant boost to your confidence.
- ⭐ Industrial-Grade Whitening. TeethWhite™ teeth whitening employs industrial-grade lamps for optimal whitening results.
- ⭐ Effective Ingredients: Our teeth whitening gel features Carbamide Peroxide, a proven and potent whitening agent.
- ⭐ Advanced technology: TeethWhite™ uses strong LED light technology to activate the whitening gel and ensure thorough and consistent results.
- ⭐ Award-Winning. Wellaholic’s treatments have been recognized by top beauty publications such as Daily Vanity, Beauty Insider, and Tropika Club Magazine.
- ⭐ Over 2000 Verified Customer Reviews. Wellaholic has over 30 industry awards and over 2000 positive reviews from customers, and >50% are repeat customers.